Scientists from the disciplines of physics and chemistry already learn during their studies that mass in a thermodynamically closed system is a conservation quantity. The law of conservation of mass, also called law of Lomonossow-Lavoisier [1], is based among other things on numerous experiments of the German scientist Heinrich Landolt [2], [3] in the late 19th century.
Various reproductions of his work followed in the course of time, e.g. by J. J. Manley with his essay “On the apparent change in weight during chemical reactions” [4]. The result was the same for all publications: No change in mass.
Until a group of German and American scientists in the eighties, who partly reproduced the Landolt experiments but partly completely redesigned them, obtained a completely different result in their experiments. Their results were partly highly significant deviations from a previously measured “zero line”, which were far above the total uncertainty of measurement [5].
Obviously, a necessary prerequisite for a clear deviation from mass constancy was the presence of a phase transition. For example, the deposition of metallic silver on the inner wall of the vessel, while the so-called Tollens’ reagent (an ammoniacal silver nitrate solution) is reduced with a 2-molar glucose solution to silver, gluconic acid, ammonia and water.
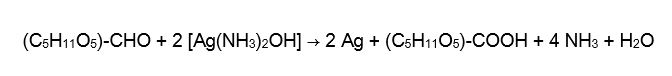
While the German-American group also investigated other forms of phase transitions in chemical, physical, and biological systems as well as in corresponding mixtures of these systems (and found significant deviations from mass constancy everywhere), we concentrate mainly on the reproduction of the silver mirror sample experiments and the Landolt experiments using modern weighing technology.
The GÖDE-Stiftung has set itself the task of investigating experiments in the field of gravitation. Of course, this also includes the question of the nature of mass and whether mass is preserved.
Since we could not find any work that tried to reproduce or at least verify the results of the German-American group, we have made it our task to find out whether such large deviations can be found again in experiments and, if so, how they can be explained, taking into account the usual regulations in metrology, such as OIML-R 111-1 [6].
With the aid of ABBA measurements on a Sartorius mass comparator (CCE36, reading accuracy 1 µg, maximum load 31 g), two test samples P1 and P3 each with a silver level sample are continuously compared against two reference samples P2 and PN (containing ground, partially sieved loess) according to the sequence N11NN22NN33N over a period of about two to three weeks.
After appropriate averaging and mass correction according to the 2007 CIPM formula [7], mass differences are determined and graphically plotted.
Should significant mass differences actually show up after completion of the various measurement series, it must be investigated which possible interference influences still occurring despite all precautions could have possibly led to these differences.
Literature:
[1] R. D. Whitaker: “An Historical Note on the Conservation of Mass”, Journal of Chemical Education, 51, 10, 658-659, 1975. [2] H. Landolt: „Untersuchungen über etwaige Aenderungen des Gesammtgewichtes chemisch sich umsetzender Körper“, Ber. Dtsch. Chem. Ges. 26, 1820 (1893). [3] H. Landolt: „Untersuchungen über die fraglichen Änderungen des Gesammtgewichtes chemisch sich umsetzender Körper. Dritte Mittheilung“, Zeitschrift für physikalische Chemie, 64U (1908) [4] J. J. Manley: On the Apparent Change in Weight during Chemical Reaction. In: Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 87, 202 (1912). [5] K. Volkamer et al.: Experimental Re-Examination of the Law of Conservation of Mass in Chemical Reactions. In: Journal of Scientific Exploration, Vol. 8, No. 2, 1994. [6] OIML R 111-1: Weights of classes E1, E2, F1, F2, M1, M1-2, M2, M2-3 and M3; Part 1: Metrological and technical requirements. International Recommendation, Edition 2004 (E). [7] A. Picard et al.: Revised formula for the density of moist air (CIPM-2007). In: Metrologia 45 (2008), 149-155.